Forest Health News No. 319 - June 2025
Welcome to the June issue of Forest Health News. In this issue, we highlight key developments across our research and biosecurity efforts—from progress in our flagship field trial to findings from a four-year ecological study in Maungatautari’s native podocarp-hardwood forest. You'll also find insights from the 2025 Biosecurity Conference and an in-depth look at the fascinating role of fungi in forest ecosystems.
We invite you to explore these stories and more as we continue advancing forest health and resilience.

Navigating Change: What the Bioeconomy Science Institute Means for Forest Health Research
Insights from Scion Principal Scientist Brian Richardson
As Heraclitus observed over two millennia ago, “The only constant in life is change.”
On 1 July 2025, the New Zealand Forest Research Institute will be incorporated into the newly formed Bioeconomy Science Institute (BSI). Depending on one’s predilection, this transformation either signals exciting new opportunities for forest health and biosecurity research—or raises significant concerns about the future role, visibility and priority of these critical activities within a broader, multi-sectoral organisation.
A Legacy of Forest Health Research
Forest health research in New Zealand predates even the establishment of the State Forest Service in 1919 (soon to be renamed the NZ Forest Service)1. However, it wasn’t until the creation of the Forest Research Institute in 1947, that dedicated teams in forest pathology and entomology were established. Since then, research activity and staffing levels have waxed and waned in response to pest outbreaks, shifting funding priorities, and broader restructures within the science system. Examples of research achievements include development of management solutions for important disease outbreaks (e.g. Dothistroma needle blight, Diplodia dieback, Nectria flute canker, red needle cast), support for pest eradication operations (e.g. painted apple moth), successful biocontrol introduction for insect pest and weed control, and contributions to improved surveillance models for pest detection1.
Some of the most significant research structure changes before the formation of BSI include the disbanding of the NZ Forest Service in 1987, the establishment of the Crown Research Institutes in 1992, and the brief Ensis joint venture with Australia’s CSIRO. The table below illustrates the evolution of forest health/biosecurity staffing over the last five decades:
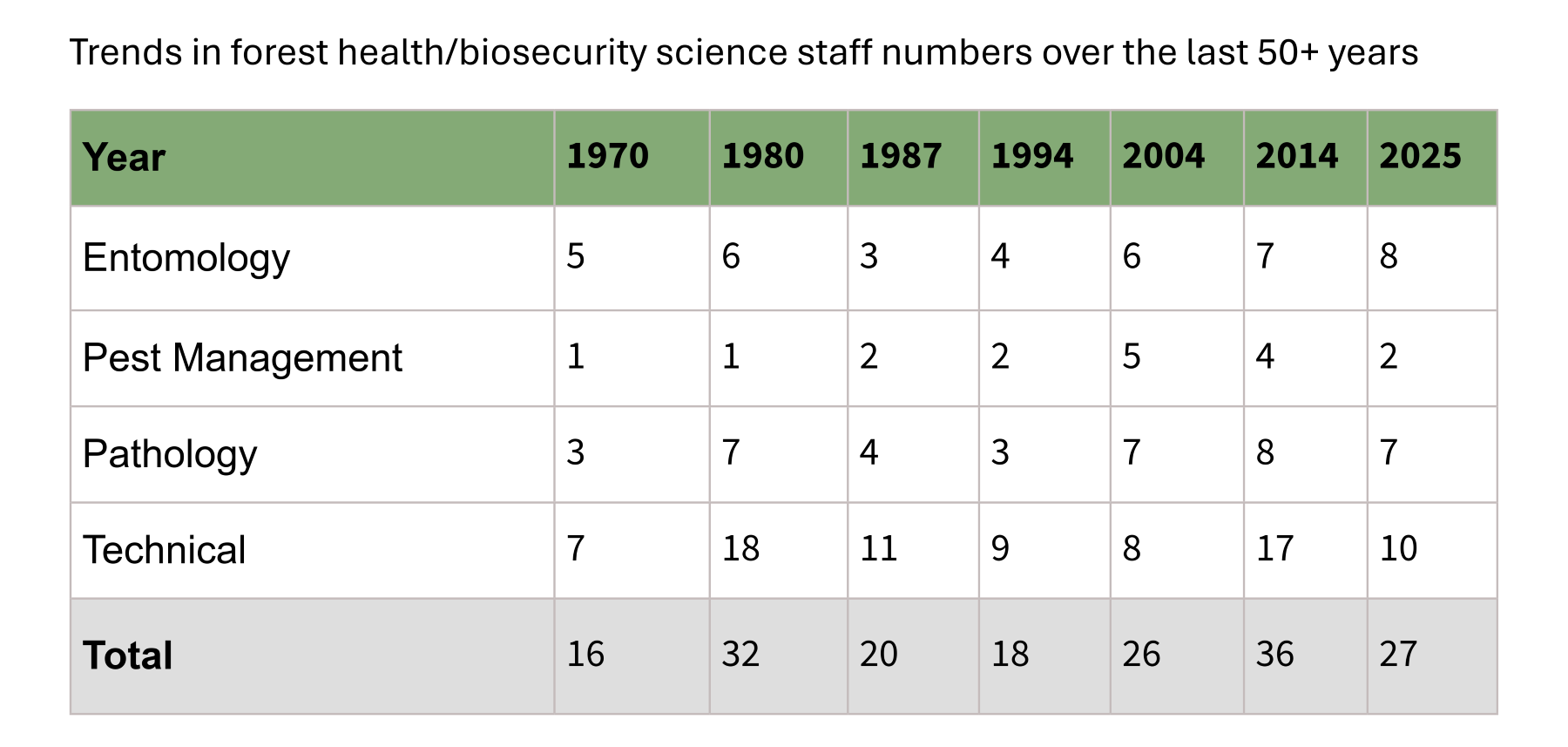
Opportunities and risks
The new Bioeconomy Science Institute offers significant potential benefits for forest health and biosecurity. It brings a broader mix of disciplines, access to enhanced facilities and technologies, and the chance to tackle forest health challenges through new partnerships and cross-sector collaboration. The Better Border Biosecurity (B3) programme has already shown the value of this kind of integrated approach but even there, institutional barriers remained. The BSI can build on B3’s strong collaborative foundation with these barriers removed and, for example, look for synergies with animal biosecurity science.However, these benefits also come with risks including the potential dilution of forest health and biosecurity as core priorities within the BSI, with attention and resources spread across other sectors. There is also the risk of losing institutional knowledge, the intimate forestry supply chain knowledge held by key researchers, as well as the vital long-standing relationships between researchers and forest managers that have underpinned effective responses to past incursions and forest health challenges1,2.
Specialised expertise in forest pathology, entomology, and pest management is not easily replaced. Ensuring these capabilities remain strong is essential for the resilience of New Zealand’s production, conservation and urban forests.
Looking ahead
To maintain a strong focus on forest health/biosecurity research in this new cross-sectoral institute, the activities must be recognised as core components of the BSI’s mission. Key steps could include:
- Strategic alignment: Ensuring the Forest Biosecurity Research Strategy clearly aligns with BSI’s overarching goals—and vice versa.
- Capability planning: Developing a specific forest health and biosecurity capability and research plan, supported by robust impact and benefit narratives illustrated by past successes.
- Sustainable funding: Ring-fencing a minimum level of investment for forest-focused biosecurity research. Industry involvement and commitments to co-funding models could strengthen the case.
- Specialist retention: Committing to maintain and grow forest health specialisations within the BSI structure.
These are foundational actions for embedding any research priority within a new organisation. But perhaps the most critical factor in the months ahead is active engagement. Forest industry stakeholders should make sure they are actively participating in the conversations and decisions that will shape the BSI’s future direction and, by extension, the direction of New Zealand’s forest health/biosecurity science.
The transition to the Bioeconomy Science Institute represents a major shift in the NZ research environment. Forest health/biosecurity research has a strong legacy to build on in this new environment—decades of specialised expertise, trusted relationships, and proven impact. With clear strategy, sustained investment, and shared commitment, the forest health/biosecurity research community can continue to safeguard New Zealand’s forests in this new era.
1 Bulman, L.; Gadgil, P. History of forest health research in New Zealand. New Zealand Journal of Forestry 2014, 59(2), 9-13.
2 Turner, J.A.; Bulman, L.S.; Richardson, B.; Moore, J.R. Cost-benefit analysis of biosecurity and forest health research. New Zealand Journal of Forestry Science 2004, 34, 324-343.

Recent activities at our flagship field trial
For fifty years the Puruki Experimental Forest, Scion’s flagship field trial, has provided valuable data that has underpinned forest management in Aotearoa/New Zealand.
Plans are underway to capture as much data as possible from harvesting (due in just under one year), and the transition to new forest systems with the potential to offer resilience to climate change and greater diversity. Insects play an important role in forest ecosystems of nutrient cycling and have close relationships to plants.
Scion entomologists Toni Withers, Mike Davy and Belinda Gresham spent the summer exploring the invertebrate life found in the forest. The team is attempting to build the most comprehensive understanding of biodiversity found in a mature plantation forest. The diversity of insect life helps us understand how healthy an ecosystem is. Each insect species feeds on particular plant parts and has a food species preference.
From December 2024 to February 2025 three light-trapping sessions were conducted in both adjacent pasture and forest sites. A total of 376 moths were collected and identified as 45 unique species, which represent approximately 15% of the North Island’s macro-moths. Of the 45 species, 41 are considered native or endemic.
The caterpillars of leafrollers, small moths that pupate in silken curled leaves, have been collected from along nine 30 m transects at Puruki from three visits during the summer. The caterpillars have been sampled from nine different host species, the most common of these being Melicytus ramiflorus, Coprosma grandifolia, and Litsea calicaris.
A total of 248 leafrollers have been brought back to the lab over the collection period. Abundance was unevenly distributed across the summer months.
Together with planned vegetation, beetle, bird and aquatic surveys the team will be able to monitor changes in species type and abundance as the forest moves through harvest to the establishment of the new forest.

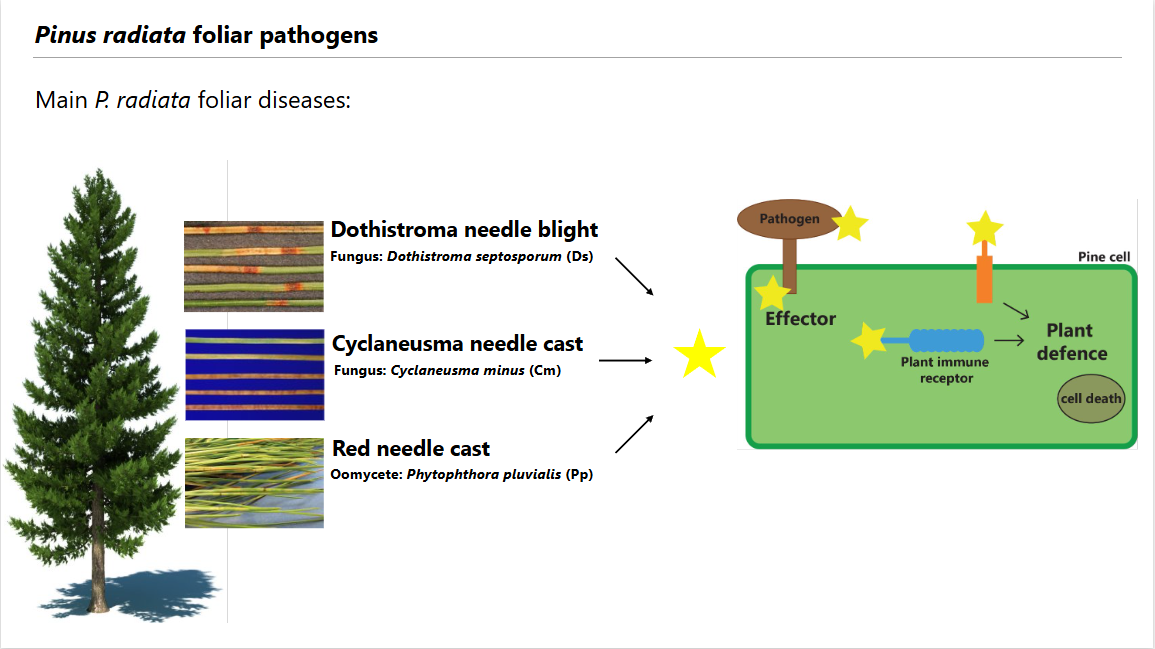
How pine trees fight back: similar proteins in diverse pathogens trigger plant immunity
Have you ever wondered how trees defend themselves against disease? Unlike us humans—who have immune cells to fight infections—plants rely on special defence proteins to recognize harmful microbes, or pathogens. A recent study reveals that some of these defence-triggering proteins used to recognise invaders are common to completely different types of pine pathogens1.
Pine trees like Pinus radiata face attacks from many pathogens. This study focused on three major threats: Dothistroma septosporum: a fungus causing Dothistroma needle blight, Cyclaneusma minus: a different fungus responsible for Cyclaneusma needle cast, and Phytophthora pluvialis: an oomycete (a fungus-like organism, but evolutionarily very different from fungi) causing red needle cast. These pathogens secrete molecules, called effector proteins, to weaken a tree’s defences and help the pathogen to colonize the plant. However, pine trees can sometimes detect these effector proteins and activate an immune response to stop the infection.

Even though these pathogens belong to different biological kingdoms, we discovered that they share similar effector proteins that can trigger immune responses in pine trees. One key protein caused cell death in pine needles, which is an extreme plant response that helps to prevent the pathogen’s ability to spread. Another effector had a similar effect, showing how the tree might use these proteins to recognize and resist attacks. Interestingly, when researchers tested these proteins on Nicotiana (a relative of tobacco and tomatoes, unrelated to pine trees), the plants also reacted, showing that these effectors can even trigger plant immunity across different plant species. This study also revealed that while some of these effectors share similar chemical compositions, others are structurally alike despite having little chemical similarity, meaning that their shapes play a crucial role in their function.
It is possible that needle pathogens not currently found in New Zealand—like Lecanosticta acicola, the cause of brown spot needle blight in other parts of the world—also possess similar proteins. Computer-based analyses have shown genes like those responsible for producing effector proteins in Dothistroma septosporum can be found in Lecanosticta acicola as well as other needle pathogens and endophytes2.
Understanding these shared effectors is exciting because it could lead to stronger, more disease resistant trees. If scientists can breed pines that recognize these common effectors, forests could naturally resist multiple diseases at once, reducing the need for chemical treatments. This study highlights the ongoing "arms race" between plants and pathogens, where both sides constantly evolve to outsmart each other. By better understanding these interactions at a molecular level, scientists are helping trees fight back.
This research was conducted by Mariana Tarallo during her PhD studies at Massey University and was funded by Scion SSIF and FGR Levy Trust funding through the Resilient Forests programme. Mariana is currently a postdoctoral fellow in BioProtection Aotearoa, at Massey University, researching Phytophthora agathidicida (kauri dieback) effector biology.
1 Tarallo, M., C.H. Mesarich, R.L. McDougal, and R.E. Bradshaw (2025). Foliar Pine Pathogens From Different Kingdoms Share Defence-Eliciting Effector Proteins. Mol Plant Pathol, 2025. 26(3): p. e70065.
2 Hunziker, L., M. Tarallo, K. Gough, M. Guo, C. Hargreaves, T.S. Loo, R.L. McDougal, C.H. Mesarich, and R.E. Bradshaw (2021). Apoplastic effector candidates of a foliar forest pathogen trigger cell death in host and non-host plants. Sci Rep, 2021. 11(1): p. 19958.
Fungi in the Forest
Forests are complex ecosystems made up of a diversity of lifeforms interacting together to their mutual benefit. Apart from trees and shrubs, which provide habitats for birds, mammals, insects and other invertebrates, there is a multitude of microscopic, unseen organisms such as bacteria and viruses all playing their part. And then there are fungi.
Fungi are key!
They fill important roles vital to the wellbeing of the healthy forest. Many are saprophytes that decompose dead organic matter, some in the leaf litter or soil layers, others (along with certain tunnelling insects) in the wood of shed branches or fallen stems and even within still living trees. This process frees up stored energy and releases bound chemical elements (carbon and essential nutrients), making them available to other organisms in the forest. Without these decomposers there would be no recycling, and the forest would eventually collapse.
Fungi reproduce by means of fruitbodies of many forms in the litter or on decaying wood. These include shelves and brackets, flat crusts, coral-like forms, toadstools, puff balls, discs, stars and a variety of other types. Another group fundamental to trees and shrubs are the mycorrhizal fungi. These infect living tree roots in a beneficial way, modifying them into ‘fungus-roots’ or mycorrhizas.
They produce webs of fungal threads, or mycelia, that penetrate through the soil in a way that enables the tree to increase the absorption and uptake of essential nutrients by its roots. Many of these are also ‘larger fungi’ that form toadstool or other conspicuous fruitbodies on the forest floor at certain times of the year. The healthy forest depends upon a balanced interaction between these various living processes facilitated by the fungi.
Although the ecology of these forest fungi has been well investigated, not least by Scion1,2, much remains to be learned. Even simply cataloguing an inventory of the species present is a valuable contribution to knowledge of the forest biodiversity, providing a benchmark for comparison with other localities, between forest types or with changes in time.
A current study looking at the larger fungi, especially those present on wood, has been running for four years in podocarp-hardwood native forest that covers Maungatautari, an imposing volcanic mountain in the Waipa District. Administered by the Maungatautari Ecological Island Trust, Sanctuary Mountain Maungatautari is approximately 3,363 hectares of forest surrounded by a predator-proof fence, allowing native wildlife to flourish free from harmful pests. Five sites with woody debris present, approximately 10-45 × 10m in size, were established in an area of forest 1.3 km across on the southern slopes of the mountain. These sites were visited 18 times over the course of the four years.
Nearly 100 species of larger fungi have now been identified and representative dried specimens lodged as voucher collections in the Scion’s National Forest Culture Collection (NZFRIM). Photographs and drawings have been made in order to record the morphological features of these and additional fungi not yet fully identified. Seasonal fruiting patterns have been noted with some species. Results have been fully documented in three reports3. This information complements and augments records lodged on the iNaturalist website as well as those made during a fungal foraging day on Maungatautari organised by Fungal Network of New Zealand (FUNNZ) during their annual foray in 2023.
The study has its limitations. Because of its focus on larger fungi with more conspicuous fruitbodies, species in other groups with microscopic fruitbodies, such as leaf spot fungi4, have mostly not been considered. Some of these may cause disease if changed conditions upset the natural balance, or if they are introduced as exotic pathogens (e.g., the myrtle rust fungus, Austropuccinia psidii). Certain species with ephemeral fruitbodies that appear infrequently are likely to be missed. Other species with perennial fruitbodies, such as some crust fungi, may be immature when sampled, lacking spores or other microscopic features necessary for precise identifications. And then, of the many fungi present in our native forests there are some that have not yet been described and named. A number of the unidentified species in this study may fall into this category.
This study examined the macro- and micromorphological features of the fungi sampled; it was not feasible to use additional procedures such as isolation of cultures and DNA sequencing. Despite these restrictions, much has already been learned during this project about the fungi on Maungatautari and it is believed that a valuable addition is being made to our knowledge of the biodiversity on the mountain reserve.
This project has benefitted by the interest and support of Dr. Janelle Ward and others of the Maungatautari Ecological Island Trust as well as iwi groups associated with Maungatautari (Ngāti Koroki-Kahukura, Ngāti Hauā, Ngāti Raukawa). The help of people from Scion is also gratefully acknowledged.
1 Addison et al. (2025): Unravelling changes in the Pinus radiata root and soil microbiomes as a function of aridity. Global Change Biology 31 (4): e70165. https://doi.org/10.1111/gcb.70165
2 Hood, I.A. (2018): Decay fungi in fallen native trees. Forest Health News 285: 1-2. Scion (New Zealand Forest Research Institute). https://cdm20044.contentdm.oclc.org/digital/collection/p20044coll2/id/210
3 Hood, I.A. (2022, 2023, 2025): Fungi on Maungatautari: larger species in native forest, especially on wood. Initial report and supplements 1 and 2 (169pp., 73pp., 79pp., resp.). Unpublished. Maungatautari Ecological Island Trust, Scion.
4 Scion (2024): Common insect pests and diseases on New Zealand native plants.



Fungal Foray based at Urenui in Taranaki
The Fungal Network of New Zealand (FUNNZ) has been organising an annual Fungal Foray that alternates between the North and South Islands since 1986. This year, the 37th foray was based in Urenui in Northern Taranaki from 11-17th May, the second foray to be held in the Taranaki region since the 13th foray in Stratford in 1999. There were 51 attendees, including Michael Bartlett and Kiryn Dobbie from Scion, with additional local participants joining in from the Taranaki Regional Council, iwi and hapū, and local conservation trusts.
Participants were able to work with New Zealand’s leading mycologists to investigate 14 different forests, parks, and reserves—where permits and permissions were obtained to make collections of fungi. One reason the annual foray is important is that physical specimens can be collected and deposited into mycological collections for further study. A significant portion of NZ’s fungal biodiversity is yet to be formally described. Collections made during the foray are representatives of species that only have ‘tag’ names and require formal descriptions (see images for examples). Foray attendees made several collections of leaf spots on native tree species that have been brought back to Scion for further research. Prior to this year’s foray, several of the locations in Northern Taranaki had no collections and essentially no information about the fungal biodiversity present in these forests. While the data from the survey is still being processed, with ongoing work to get DNA sequences and describe specimens, observations uploaded to iNaturalist during the week give some idea of what was found – in total 790 observations of approx. 192 different fungi were made!
Amid the hustle and bustle of the foray, the 23rd Mycology Colloquium was held at the Urenui community centre. Michael Bartlett spoke about survival of poplar rust (Melampsora Iaricis-populina) spores against ultraviolet light in the upper atmosphere, and Kiryn Dobbie presented the results of inoculation studies with the tōtara blight pathogen, Phytophthora podocarpi. A variety of interesting research was presented ranging from talks about mycorrhizal Cortinarius and Pisolithus fungi, psychoactive psilocybin mushrooms, sooty molds, fungal taxonomy and type specimens, the historical trading of wood ear mushrooms in Taranaki, and the historical paintings of fungi in Taranaki made by Fanny B. Good (1860-1950).


Terminal Crook: Can you spot the difference?
Terminal crook disease, caused by the fungus Colletotrichum acutatum, has re-emerged as a damaging issue in commercial pine nurseries across Aotearoa New Zealand. It has been reported from several locations, including the North Island and the Nelson region. The disease targets the growing tips of pine seedlings, causing them to bend into a characteristic stiff crook. In severe cases, the apical shoot dies off, leading to stunted growth.
As part of a terminal crook disease research programme led by Scion, Forest Growers Research, and members of the New Zealand Forest Nursery Growers Association, the Forest Health Reference Laboratory has received multiple seedling samples with suspected symptoms. However, not all cases have turned out to be Colletotrichum! Other common nursery pathogens can cause similar symptoms and are easily mistaken for terminal crook disease.
These include the grey mould Botrytis cinerea, as well as Phytophthora and Fusarium species, which can rot roots and collars, leading to wilting of the apical tip. In stressed seedlings, fungi such as Diplodia sapinea (blue stain fungus) have also been isolated from dying or wilting shoots. Other fungal pathogens not listed here may also cause similar symptoms, making accurate diagnosis essential.
Misidentifying the cause of wilting can result in ineffective or unnecessary chemical treatments—costing time, money, and potentially compromising seedling health.
Scion’s Forest Health Reference Laboratory offers a diagnostic service to help nursery managers identify the cause of symptoms and make better-informed management decisions. If you’re seeing dieback or crooking in your seedlings, don’t assume it’s terminal crook—get a diagnosis instead.
-
Terminal crook

Typical terminal crook symptoms caused by Colletotrichum acutatum.
-
Fusarium root rot

Fusarium root rot caused by Fusarium spp. can cause the tips to wilt.
Investing in Forest Health
Insights from the 2025 Biosecurity Conference
In April, industry stakeholders from across Aotearoa gathered at the University of Canterbury in Ōtautahi Christchurch for the 2025 Forest Biosecurity Conference. The two-day event showcased how innovation and technology are shaping biosecurity research and delivering real-world impact to safeguard the health of New Zealand’s forests.
The conference opened with the joint Biosecurity New Zealand / Forest Owners Association biosecurity forum—an interactive session providing updates on government and industry initiatives. Facilitated by Peter Thomson (Biosecurity New Zealand) and Mike Lawson (GIA Partnerships), the forum fostered open dialogue on past achievements and future plans. These conversations are vital for ensuring transparency and empowering stakeholders to help shape the future of their industry.
This year’s keynote address was delivered by Dr Angus Carnegie, a leading forest health scientist from New South Wales, Australia. Dr Carnegie reflected on the evolution of forest health protection throughout his career—from helicopter surveillance to satellite imagery, and from morphological identification to environmental DNA (eDNA) monitoring. While technology is advancing rapidly, he emphasised that one of the most enduring challenges is maintaining a skilled workforce. With a dwindling pool of forest health experts, he called for greater investment in people and in building the skills and capabilities needed to sustain research excellence.
International perspectives also featured prominently. A panel discussion highlighted insights from a 2024 forest biosecurity tour to the Basque Country, Spain. Participants—including Steve Gatenby (Kaingaroa Timberlands), Dr Rebecca McDougal (Scion), and Phil Taylor (Port Blakely)—shared first-hand accounts of the challenges European forest growers face, such as Lecanosticta acicola (brown spot needle blight), and the implications for New Zealand. The tour left a strong impression on the need for greater investment and improved readiness for emerging threats.
Closer to home, the conference celebrated the value of local research and the investments that make it possible. Alison Slade (Forest Growers Research) reflected on how past research has shaped current best practices, highlighting the need to invest beyond the research phase to ensure science delivers impact. Meanwhile, Dr Beccy Ganley (Plant & Food Research and Kaihautū Tiriti of the Better Border Biosecurity [B3] programme) emphasised the long-term value of sustained research investment. B3, which marks its 20th anniversary in 2025, remains a cornerstone of biosecurity innovation in Aotearoa New Zealand.
Throughout the conference, participants engaged in sessions on a wide array of topics: safeguarding native biodiversity and ngahere health, using eDNA for early detection, tracking plant movement, aerial surveillance technologies, breeding trees for disease resilience, and harnessing beneficial microbes to prevent infection. Presentations also explored social and behavioural dimensions—such as citizen science and app-based reporting—and the importance of understanding pest pathways and risk perception to strengthen response.
While many talks focused on specific diseases or technologies, a recurring theme was that forest health is a dynamic and evolving landscape. With new pathogens emerging and complex interactions between climate change, trade, and human movement, our forests face threats from multiple—and sometimes unknown—causal agents. This reality reinforces the importance of investing in people and research, fostering cross-sector collaboration, embracing adaptive management, and maintaining vigilance across the biosecurity system.
We look forward to taking part in the next Forest Biosecurity Conference in 2026 as we continue to share, collaborate, and innovate to protect our forests.

Spotlight on fall armyworm in Aotearoa
In a bid to protect New Zealand's forests and agriculture, researchers are intensifying efforts to monitor fall armyworm, Spodoptera frugiperda, a pest first detected in the country in 2022. This invasive species, known for its migratory prowess, has spread from the Americas to Africa, Asia, Australia, and now New Zealand. Predicted to be a significant threat to maize it has so far been observed mainly in Taranaki.
The Protecting Aotearoa from wind-dispersed pests research programme, funded by the MBIE Endeavour Fund, along with Te Uru Kahika and the Foundation for Arable Research (FAR), is deploying automated scouting traps to monitor these pests. These traps use pheromones to attract male moths and AI technology to identify and report findings.
Successful monitoring will help inform pest spread predictions, alert maize growers, and corroborate climate models, ensuring proactive measures to safeguard New Zealand's biosecurity.
Forest Health Snippets
- Call for input: Are you a forest owner or manager dealing with needle disease in your conifer stands? Scion is drafting a “Red Needle Cast Handbook” and is seeking input from foresters. What questions do you have about RNC, how to identify it, what to do about it? No question too big or too small! Contact FHN editor Hazel Daniels with questions or comments about what you’d like to see in a RNC Handbook tailored for use in the bush.
- Upcoming event: The 2025 Australasian Myrtle Rust Conference will be held from 16-17 June in Auckland. Talks will be livestreamed. If you are interested in watching the conference virtually you can find more information on the conference website.
- New technical guide: Fresh off the press! “Sampling to detect soilborne Phytophthora infestations in California habitat restoration plantings: a technical guide” is a new document released by the U.S. Forest Service to help land managers identify sites affected by root-rotting Phytophthora species. While the guide is limited in scope to California restoration plantings, the topics and strategies discussed are applicable to a much wider audience. A downloadable PDF is available on the USFS website at https://doi.org/10.2737/psw-gtr-279.
- New plant species: A newly described plant species in South Africa—Delosperma buysii—has been named in honour of Matt Buys, Scion’s herbarium curator. This recognition highlights Matt’s lasting impact on botanical science and biodiversity, and his efforts to strengthen public understanding of the essential role plants and forests play in a circular bioeconomy. You can read the paper describing the new species here: https://phytotaxa.mapress.com/pt/article/view/phytotaxa.635.3.5.
- Want more news? Are you interested in what else Scion is working on? Subscribe to several newsletters focusing on different aspects of the work we do to improve New Zealand’s forests here: Scion.
- Want more news? Have you heard about the “Protecting Aotearoa from wind-dispersed pests” programme? Subscribe to Blown Away newsletter signup
